This product is no longer manufactured. Remaining stock may still be available. Please refer to the alternatives listed below under "Similar products" or contact us directly.
Alternative Products for this discontinued product:
XD-9502
Photostability Light & UV Meter
UV-A and lux detector head for drug photostability test measurements
- UV-A irradiance and Illuminance sensors
- For use with X1 type optometers optimized
- Including calibration certificate

The term photostability refers to the ability of a substance or material to remain unchanged under exposure to light. Light as a form of electromagnetic radiation is capable of affecting chemical changes in some substances over time. The pharmaceutical industry must take special precautions to ensure that their drug products and packaging are not adversely affected by light exposure.
"ICH" the International Conference on Harmonization for the pharmaceutical industry publishes guidelines for testing photostability of drugs and drug products.
There are two options for exposing samples to UV, primarily UVA, and white light. Measuring and monitoring the UV irradiance and light levels is necessary to ensure stated exposure (dose) levels for both UVA and light are achieved.
Exposing Option 1
Any light source that is designed to produce an output similar to the D65/ID65 emission standard such as an artificial daylight fluorescent lamp combining visible and ultraviolet (UV) outputs, xenon, or metal halide lamp. D65 is the internationally recognized standard for outdoor daylight as defined in ISO 18909:2022 (formerly ISO 10977 :1993). ID65 is the equivalent indoor indirect daylight standard. For a light source emitting significant radiation below 320 nm (nanometers), an appropriate filter(s) may be fitted to eliminate such radiation.
Exposing Option 2
For option 2 the same sample should be exposed to both the cool white fluorescent and near ultraviolet lamp.
- A cool white fluorescent lamp designed to produce an output similar to that specified in ISO 18909:2022 (formerly ISO 10977 :1993); and
- A near UV fluorescent lamp having a spectral distribution from 320 nm to 400 nm with a maximum energy emission between 350 nm and 370 nm; a significant proportion of UV should be in both bands of 320 to 360 nm and 360 to 400 nm.
Also with the UVA sensor flat spectral response and the high fidelity to CIE V(λ) function lux sensor both Option 1 and 2 lamp types can be measured with the same unit without changing calibration factors. For future considerations this would also apply to LED based white light and UV-A sources.
Both detectors have a cosine corrected field of view for accurate spatial responsivity.
XD-9502-4 Photostability Light and UV Meter
The light sensor recommended for photostability light exposure testing is the XD-9502-4 dual cell low profile detector that houses both UV-A and lux detectors so both measurements can be made simultaneously. This means no fumbling, no repositioning of the detector for faster measurements and mapping of the exposure plane.
Also with the UVA sensor flat spectral response and the high fidelity to CIE v-lambda function lux sensor both Option 1 and 2 lamp types can be measured with the same unit without changing calibration factors. The detector shows a cosine-corrected field of view.
For Use With: X1-1 Four-Channel USB Radiometer/Photometer
Internationally Traceable Calibration
All filter detectors are calibrated to internationally traceable standards following ISO 17025 guidelines and requirements. Gigahertz-Optik optical radiation calibration laboratory is DIN EN ISO/IEC 17025 accredited (DaKKs Registration No. D-K-15047-01-00).
Application Case:
Here's the problem
You are responsible for toxicity testing of your drug products and drug packaging under light exposure according to ICH Q1B Photostability Testing of New Active Substances and Medicinal Products. ICH states that light testing should be an integral part of stress testing of pharmaceuticals and its packaging. The intrinsic photostability characteristics of new drug substances and products should be evaluated to demonstrate that, as appropriate, light exposure does not result in unacceptable change.
Here are the requirements
According to the guidelines two types of light sources as already described as options 1 and 2 can be used for photostability testing. A pharmaceutical manufacturer/applicant may rely on the spectral distribution specification of the light source manufacturer. A simple and accurate means of measuring UVA and visible light inside the photostability exposure chamber is required for both lamp types.
Here's the solution
The X1-1 + XD-9502-4 Photostability Meter can be used for both lamp types for Options 1 & 2 of the ICH Q1B guidelines.
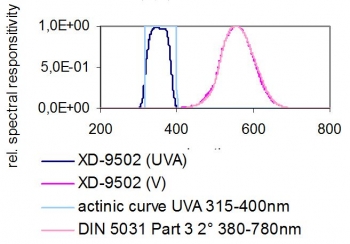
XD-9502-4 UV-A Sensor and V(λ) Sensor - Typical Spectral Responsivities
Similar Products
Product Categories

